Introduction
Indolent Non-Hodgkin lymphomas (NHL) comprise a heterogeneous group of diseases including marginal zone lymphoma (MZL), lymphoplasmacytic lymphoma (LPL), small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL), and follicular lymphoma (FL). These compose a heterogenous group of disorders that frequently measures survival in years due to the long natural history of these diseases. Frequency and morbidity of cardiac arrhythmias in patients with indolent lymphoma is unknown, but recent observations note that arrhythmias are an increasing problem. Due to advances in treatment for indolent NHL with emergence of novel therapeutics, combined with an aging population and a long natural history, understanding of arrhythmia burden in indolent lymphoma is an area of research with important implications for patients undergoing active treatment as well as for long term lymphoma survivors.
Methods
Adult patients 18 years or older with indolent NHL treated at the University of Rochester Wilmot Cancer Institute between 2013-2019 were included in the Cardio-Oncology Lymphoid Malignancies Database and analyzed. The primary objective of this study was to define the rate of arrhythmic events and sudden cardiac death in patients with indolent lymphoma during treatment. Cardiac arrhythmias including ventricular arrhythmias (VT/VF), atrial arrhythmias (atrial fibrillation (afib), flutter, SVT and atrial tachycardia), and bradyarrhythmias were identified using ICD-10 codes. Kaplan-Meier survival analysis was used to assess cumulative probability of arrhythmia.
Results
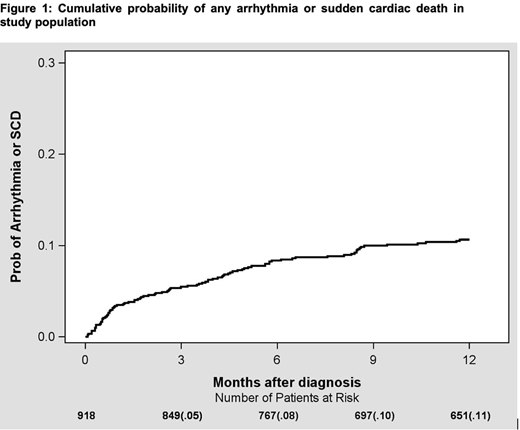
There were nine hundred and eighteen patients who were diagnosed with indolent lymphoma. Diagnoses included: CLL, N=414; FL, N=284; MZL, N=144; LPL, N=76. Median age was 64, and 43% were female. There were 383 (42%) patients who received treatment. Treatments were classified as chemotherapy, targeted therapy, monoclonal antibodies/immunotherapy, and combination therapy. There were no significant differences in baseline characteristics between treated and never treated patients. At the time of diagnosis, 277 patients (30%) had hypertension, 101 (11%) had prior history of arrhythmia. During median follow up of 24 months, 168 patients (18%) developed a new or recurrent arrhythmia based on ICD-10 codes documented in the electronic medical record. Sixty-three out of one hundred sixty-eight patients had both prior history of and recurrence of arrhythmia, while one hundred five had a new diagnosis of arrhythmia. Afib was the most common arrhythmia, noted in 81 patients (9%). At 6 months from diagnosis, cumulative probability of developing any arrhythmia was 8% (Figure 1). Of all arrhythmias, 89/168 (53%) occurred in SLL/CLL, 35/168 (21%) in FL, 17/168 (10%) in LPL, 27/168 (16%) in MZL. Arrhythmias on treatment occurred in 4/95 patients receiving chemotherapy alone (4.2%), 12/95 patients receiving monoclonal antibodies/immunotherapy (12.6%), and 28/95 patients receiving targeted therapy (29.4%). Most arrhythmias (51/95; 53.6%) occurred in patients receiving combination therapy (chemoimmunotherapy or targeted/immunotherapy). Overall, there were 80 (9%) deaths. Ten deaths were related to cardiovascular diseases; of which 8/10 (80%) were from sudden cardiac death.
Conclusions
This real-world cohort demonstrates that patients with indolent lymphoma could have an increased risk of cardiac arrhythmias that is increased by treatment. Afib was the most common arrhythmia identified in this study and appears increased compared to the incidence in the general age matched population (1-1.8 per 100 person-years). Surprisingly, of 80 deaths, 8 (10%) were attributed to sudden cardiac death. This data set contributes important information that can help identify patients at increased risk of cardiovascular morbidity and mortality that can impact treatment. Prospective monitoring in these patients may better define the incidence and associated risks of arrhythmias. Future directions will focus on risk factors for arrhythmias and developing an approach to prevent and treat arrhythmias in this patient population. Updated results will be presented at the meeting.
Zent:Acerta / Astra Zeneca: Research Funding; TG Therapeutics, Inc: Research Funding; Mentrik Biotech: Research Funding. Barr:Janssen: Consultancy; Abbvie/Pharmacyclics: Consultancy, Research Funding; Verastem: Consultancy; Celgene: Consultancy; Seattle Genetics: Consultancy; TG therapeutics: Consultancy, Research Funding; Morphosys: Consultancy; Gilead: Consultancy; AstraZeneca: Consultancy, Research Funding; Merck: Consultancy; Genentech: Consultancy. Reagan:Kite, a Gilead Company: Consultancy; Seattle Genetics: Research Funding; Curis: Consultancy. Friedberg:Seattle Genetics: Research Funding; Roche: Other: Travel expenses; Bayer: Consultancy; Astellas: Consultancy; Acerta Pharma - A member of the AstraZeneca Group, Bayer HealthCare Pharmaceuticals.: Other; Kite Pharmaceuticals: Research Funding; Portola Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.